
















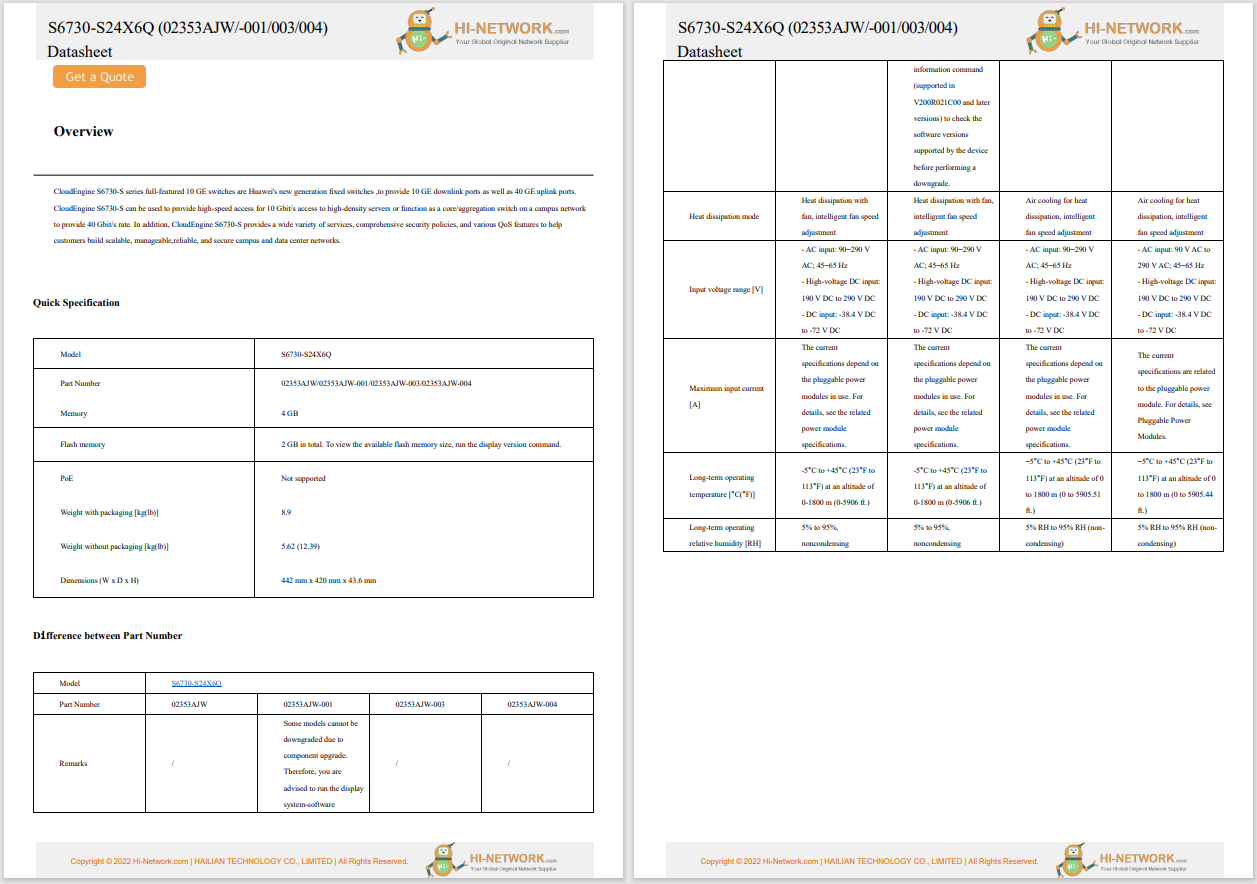
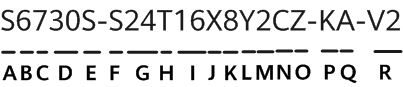












A bill aimed to provide for the regulation, inspection, and labelling of food produced using animal cell culture technology was introduced in the US House of Representatives. Titled 'Food Safety Modernization for Innovative Technologies Act', the bill builds on an agreement established in early 2019 between the US Food and Drug Administration (FDA) and the US Department of Agriculture (USDA) on the regulation of the development and sale of cell-cultured meat products. According to the bill, the FDA would oversee the initial cell collection, proliferation, and culturing processes, while the USDA will have regulatory oversight over the processing and packaging of the harvested cells and the resulting products. USDA would also have exclusive authority over labelling requirements for cell-cultured meat products derived from cell lines of livestock or poultry, as well as responsibility for establishing 'appropriate nomenclature' for these product labels. The bill also requires the FDA and USDA to share information and collaborate during cell differentiation and harvesting. The same bill was introduced in the US Senate in December 2019.
 Hot Tags :
Emerging technologies
Hot Tags :
Emerging technologies