







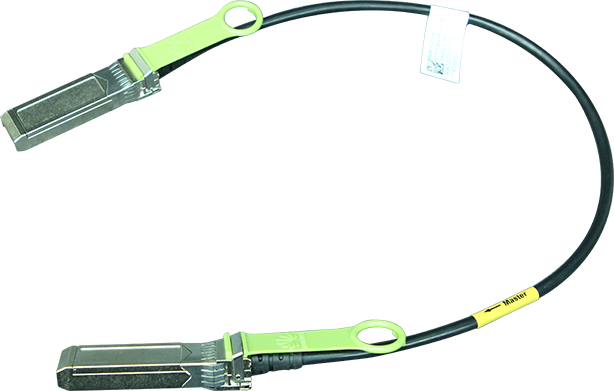







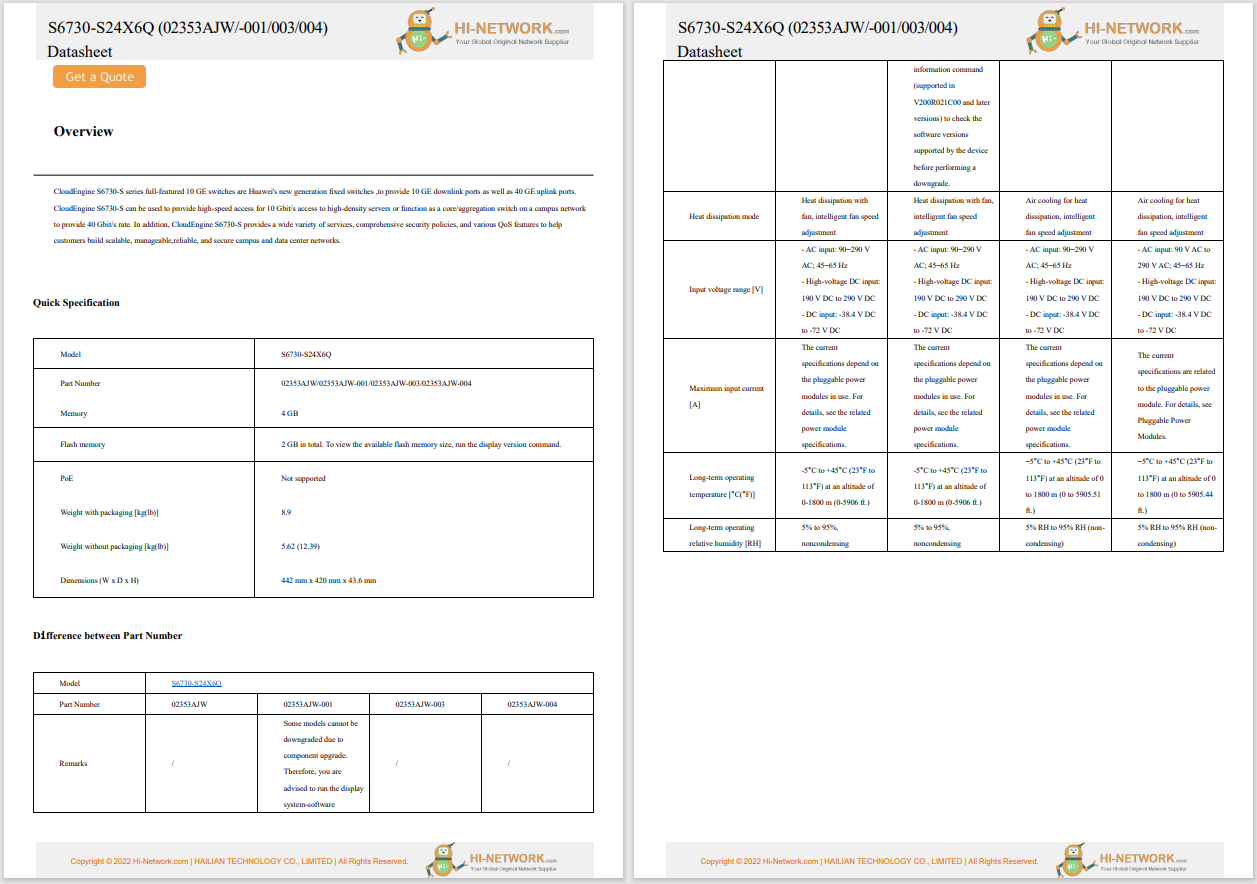
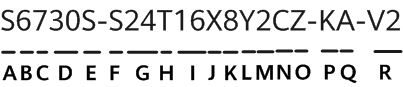















According to NextGov, the US Department of Health and Human Services (HHS) has relaxed restrictions on the collection and protection of patient health data regarding COVID-19.In a notification, the HHS stated that the HHS Office for Civil Rights (OCR) will not impose penalties for noncompliance, with the regulatory requirements under the HIPAA Privacy Rules, against covered healthcare providers or their business associates in connection with the good-faith participation in the operation of COVID-19 Community-Based Testing Sites (CBTS) during the COVID-19 nationwide public health emergency. The notification of enforcement discretion became effective on 9 April 2020, and had a retroactive effect to 13 March 2020. It will remain in effect until the Secretary of HHS declares that the public health emergency no longer exists, or upon the expiration date of the declared public health emergency.
 Hot Tags :
Privacy and data protection
Hot Tags :
Privacy and data protection
Register Email now for Weekly Promotion Stock
100% free, Unsubscribe any time!
Add 1: Room 605 6/F FA YUEN Commercial Building, 75-77 FA YUEN Street, Mongkok KL, HongKong Add 2: Room 405, Building E, MeiDu Building, Gong Shu District, Hangzhou City, Zhejiang Province, China
Whatsapp/Tel: +8618057156223 Tel: 0086 571 86729517 Tel in HK: 00852 66181601
Email: [email protected]
 English
English Pусский
Pусский Français
Français Español
Español Português
Português