






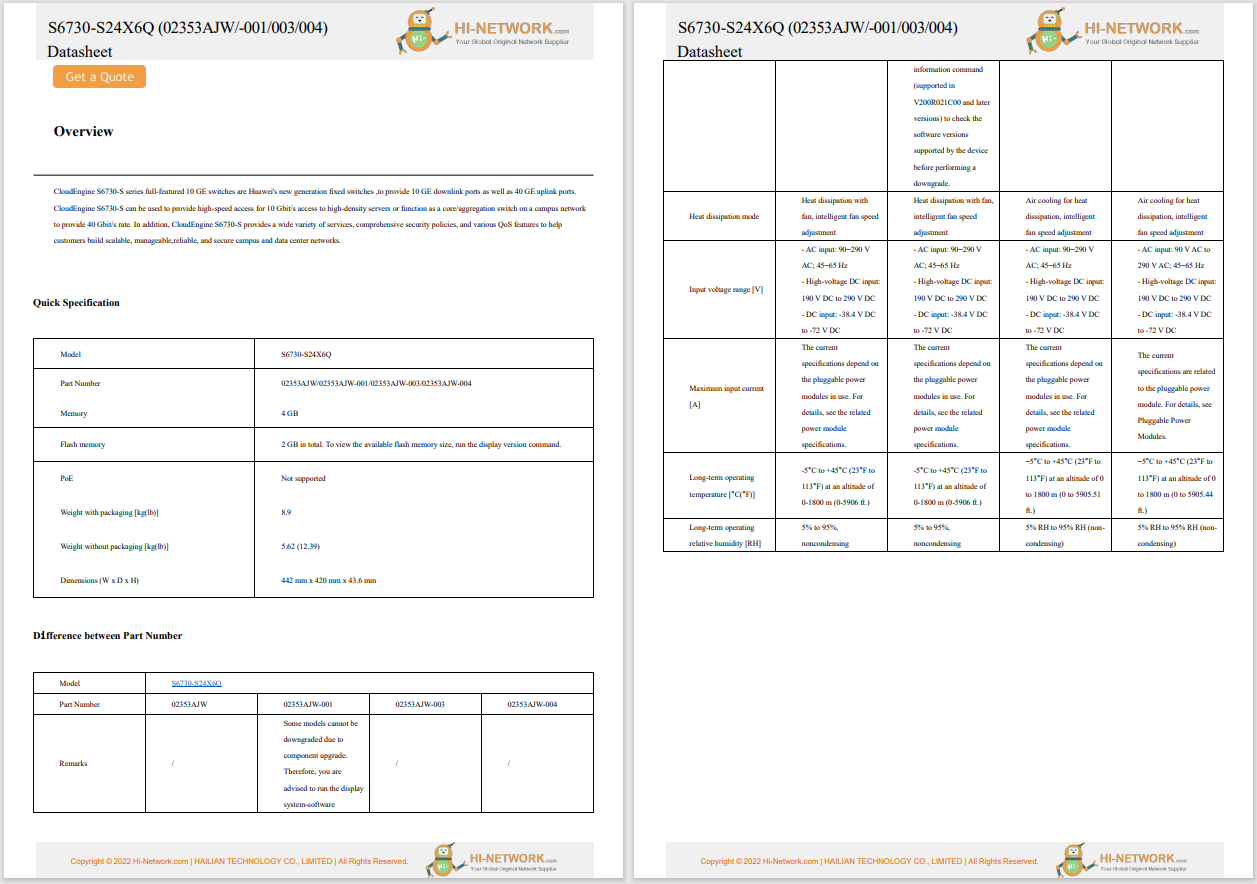





















The International Commission on the Clinical Use of Human Germline Genome Editing has issued a set of recommendations on heritable human genome editing (HHGE), noting that the technique raises scientific and medical concerns, as well as ethical, moral, and societal issues. The commission is calling on scientists not to 'attempt to establish a pregnancy with a human embryo that has undergone genome editing unless and until it has been clearly established that it is possible to efficiently and reliably make precise genomic changes without undesired changes in human embryos'. It also notes that an extensive societal dialogue should be held before a country decides on whether to permit clinical use of HHGE. Should a country permit such technique, initial uses should be limited to diseases that cause severe morbidity or premature death. Moreover, the country should have mechanisms and competent regulatory bodies in place to ensure that relevant conditions are met for the clinical use of HHGE. The commission also recommends the establishment of an International Scientific Advisory Panel to, among other tasks, assess whether preclinical requirements have been met for any circumstances in which HHGE may be considered for clinical use.
 Hot Tags :
Emerging technologies
Hot Tags :
Emerging technologies