






















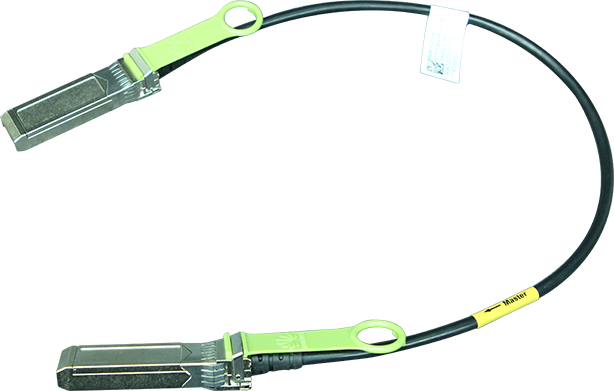







The US Department of Commerce and the Department of Health and Human Services have launched a 120-day pilot programme to fight the illegal online sale of unapproved opioids. Within this programme, the National Telecommunications and Information Administration (NTIA) and the Food and Drug Administration (FDA) will work with three domain name registries, Neustar (managing .us), Verisign (managing .com and .net) and the Public Interest Registry (managing .org) to suspend the domain names of websites found to be illegally selling unapproved opioids. The FDA will act as a 'trusted notifier' and alert the registries about websites that are illegally selling unapproved opioids; registries may voluntarily lock, delete, or place the domain on hold, as appropriate.
 Hot Tags :
Content policy
Critical internet resources
Hot Tags :
Content policy
Critical internet resources
Register Email now for Weekly Promotion Stock
100% free, Unsubscribe any time!
Add 1: Room 605 6/F FA YUEN Commercial Building, 75-77 FA YUEN Street, Mongkok KL, HongKong Add 2: Room 405, Building E, MeiDu Building, Gong Shu District, Hangzhou City, Zhejiang Province, China
Whatsapp/Tel: +8618057156223 Tel: 0086 571 86729517 Tel in HK: 00852 66181601
Email: info@hi-network.com
 English
English Pусский
Pусский Français
Français Español
Español Português
Português